Bending elasticity of anti-parallel β-sheets
Abstract
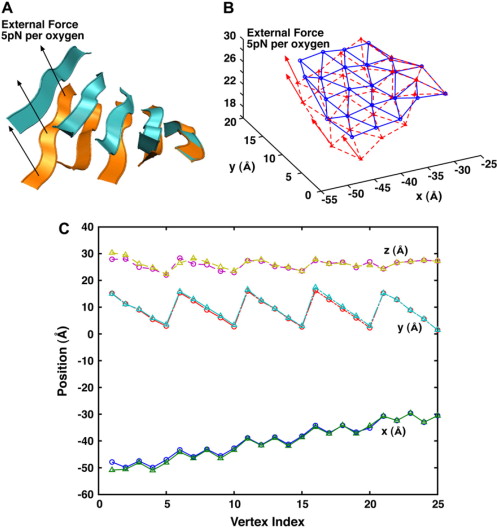
Using a coarse-grained elastic model, we examine the bending properties of anti-parallel β-sheets comprised of uniform amino-acid residues in vacuum as well as in explicit solvent. By comparing the conformational probability of the β-sheet from molecular dynamics simulations with the same quantities obtained from the coarse-grained model, we compute the elastic bending constant, κ. Equilibrium fluctuations of the β-sheet and its response to external forces are well reproduced by a model with a uniform isotropic bending constant. An anisotropic bending model is also investigated, although the computed anisotropy is relatively weak and most of the observed properties are well described by an isotropic model. The presence of explicit solvent also lowers the bending constant. The sequence dependence of our result and its implications in protein conformational dynamics are discussed.