Decoding diluent-driven solvation dynamics in locally concentrated ionic liquid electrolytes
Abstract
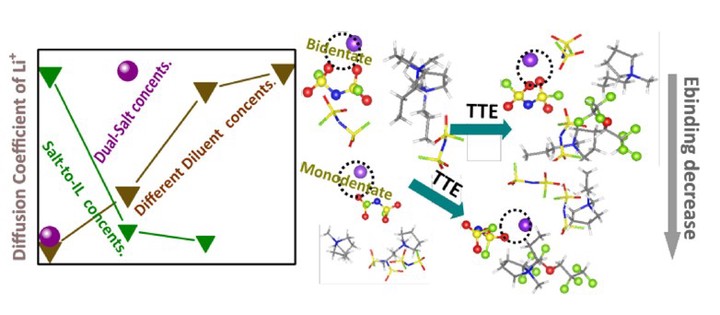
The interplay between lithium salts and anions critically influences the electrochemical and physicochemical properties of locally concentrated ionic liquid electrolytes (LCILEs), a promising class of materials for next-generation lithium-ion batteries. Here, we investigate how varying diluent concentrations modulate lithium–anion interactions, ion dynamics, and transport properties in LCILEs. Using molecular dynamics (MD) simulations combined with density functional theory (DFT) calculations, we show that incorporating 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropyl ether (TTE) as a diluent minimally perturbs the solvation structure while effectively weakening Li+–anion interactions and promoting ionic dissociation. Adjusting the lithium salt-to-ionic liquid (IL) ratio alters the coordination environment of bis(fluorosulfonyl)imide anions (FSI–). It reduces the presence of pyrrolidinium cations in the lithium solvation shell. Beyond solvation effects, we further demonstrate that introducing lithium hexafluorophosphate (LiPF6) enhances ionic conductivity and increases the lithium-ion diffusion coefficient. By systematically exploring the impacts of diluent concentration and ionic additives, our theoretical framework offers molecular-level insights into how electrolyte composition influences lithium-ion mobility and interfacial stability, key factors in designing high-performance electrolytes for next-generation energy storage systems.